
Comprehensive Guide to Type 2 Diabetes: Holistic and Functional Medicine Approaches for Management and Reversal
Type 2 diabetes is a metabolic condition driven primarily by insulin resistance that often leads to sustained high blood glucose. This guide breaks down the biology, the tests that matter (HbA1c, fasting glucose, OGTT, CGM), and the evidence-based, natural strategies that help improve blood sugar control — and in many cases support remission. You’ll learn how insulin resistance develops, which upstream drivers beyond glucose (inflammation, gut imbalance, hormonal disruption, oxidative stress) worsen the disease, and which practical steps—diet plans, exercise prescriptions, sleep and stress fixes, and targeted supplements—support metabolic recovery and symptom relief. We include clear diet and supplement comparisons, clinician-friendly tables to weigh options, and monitoring templates you can use to set timelines and goals. Finally, the guide explains how a functional medicine practice personalizes care to address root causes, layer advanced diagnostics, and combine nutrition, hormones, supplements, and supportive therapies for measurable improvement in metabolic health.
What Is Type 2 Diabetes and How Is It Diagnosed?
Type 2 diabetes is a chronic disorder in which tissues become less responsive to insulin, producing a relative insulin shortfall and persistent elevations in fasting and post-meal glucose. The process starts with impaired insulin signaling in muscle, liver, and fat tissue, which raises hepatic glucose output and lowers peripheral glucose uptake — changes that show up as higher HbA1c and fasting plasma glucose. Detecting dysglycemia early matters: weight loss and interventions that restore insulin sensitivity reduce complications and can sometimes lead to remission. In practice, diagnosis and staging rely on glycemic thresholds, symptom assessment, and individual risk factors to guide how often we monitor and which treatments to prioritize.
What Are the Common Symptoms and Risk Factors of Type 2 Diabetes?
Symptoms of type 2 diabetes usually appear slowly and reflect chronic high blood sugar and its effects on organs and tissues. Typical signs include increased urination, persistent thirst, unexplained weight changes, ongoing fatigue, blurred vision, and wounds that heal slowly; neuropathic pain or numbness may develop later. Risk factors include non-modifiable items—age over 45, family history, certain ethnic backgrounds—and modifiable contributors such as excess weight, inactivity, poor diet, and smoking. Spotting modifiable risks early lets clinicians and patients focus on prevention strategies — primarily weight loss, increased activity, and dietary change — to lower the chance that prediabetes progresses to diabetes.
- The most common symptoms of Type 2 diabetes include:
Increased urination (polyuria): Excess glucose pulls water into the urine, increasing frequency.
Excessive thirst (polydipsia): Fluid losses trigger persistent thirst and risk of dehydration.
Fatigue and blurred vision: Elevated glucose interferes with cellular function and lens hydration.
Slow-healing wounds and recurrent infections: High glucose impairs immune response and tissue repair.
Unexplained weight loss or gain: Metabolic shifts change appetite and fat storage.
Numbness or tingling in extremities: Peripheral neuropathy can develop over time.
These signs prompt clinicians to order diagnostic testing and to prioritize interventions that target reversible drivers of insulin resistance.
How Is Type 2 Diabetes Diagnosed Through Medical Tests?
Diagnosis rests on standardized glycemic tests whose thresholds determine diagnosis, staging, and monitoring. The main tools are HbA1c, fasting plasma glucose (FPG), and the 2-hour oral glucose tolerance test (OGTT); continuous glucose monitoring (CGM) is increasingly used to reveal daily patterns and variability. Each test has pros and cons: HbA1c gives a 2–3 month average but can be skewed by hemoglobin variants or anemia; FPG is simple but may miss post-meal spikes; OGTT is sensitive for early dysglycemia but less convenient in routine practice.
| Test | Diagnostic Thresholds | Notes |
|---|---|---|
| HbA1c | ≥6.5% diagnostic; 5.7–6.4% = prediabetes | Reflects long-term average glucose; results may be affected by some blood disorders |
| Fasting Plasma Glucose | ≥126 mg/dL diagnostic; 100–125 mg/dL = prediabetes | Easy and widely available; can miss postprandial hyperglycemia |
| 2-hour OGTT | ≥200 mg/dL diagnostic; 140–199 mg/dL = impaired glucose tolerance | More sensitive for early abnormalities; more burdensome for the patient |
Monitoring frequency typically ranges from quarterly HbA1c checks while control is changing to every six months once stable. CGM is a useful tool for medication adjustment, evaluating post-meal excursions, and tailoring lifestyle changes.
What Are the Root Causes of Type 2 Diabetes from a Functional Medicine Perspective?
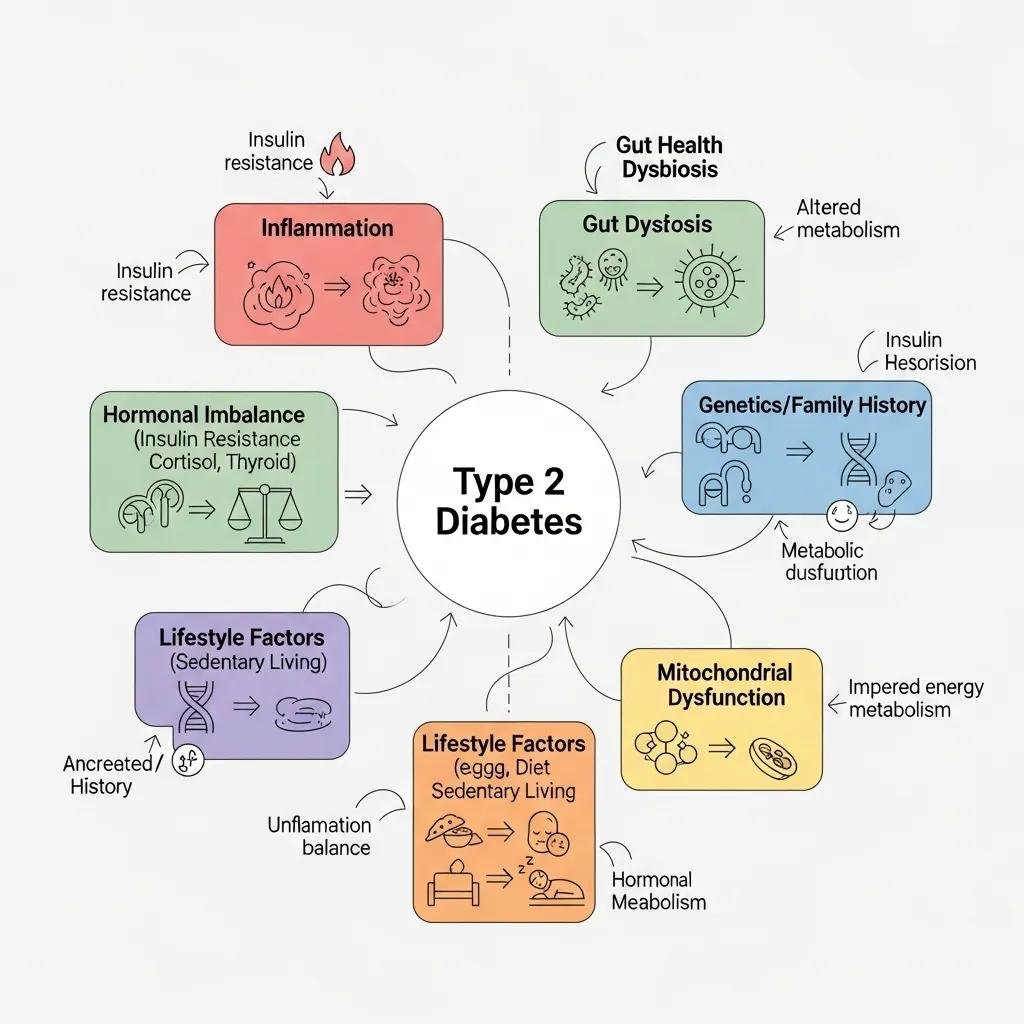
From a functional medicine viewpoint, type 2 diabetes is the downstream outcome of multiple interacting upstream drivers that disrupt insulin signaling and metabolic balance. Common root causes include chronic, low-grade inflammation, gut microbiome imbalances, hormonal dysregulation (sex hormones, thyroid, cortisol), oxidative stress, environmental toxin exposure, and nutrient insufficiencies. Each of these alters insulin signaling or energy metabolism through identifiable pathways. By testing for and addressing these contributors with targeted, evidence-informed interventions, clinicians can reduce glycemic burden and strengthen metabolic resilience. Mapping root causes to specific therapies helps prioritize treatments that restore physiology instead of only treating symptoms.
How Do Inflammation and Gut Health Influence Diabetes Development?
Inflammation and gut dysbiosis strongly influence metabolic control by changing immune signaling, gut permeability, and microbial metabolites that affect insulin sensitivity. Pro-inflammatory cytokines (for example, TNF-α and IL-6) impair insulin receptor signaling, while translocation of endotoxin (LPS) from a leaky gut can chronically activate innate immunity and promote inflammation in fat tissue. Improving gut health—by increasing fiber, supporting prebiotic and probiotic foods, and removing dietary inflammatory triggers—promotes production of short-chain fatty acids and often improves insulin sensitivity. Stool testing and inflammatory markers can help identify actionable gut-related drivers; treating them frequently correlates with better glycemic measures.
What Role Do Hormonal Imbalances and Oxidative Stress Play in Insulin Resistance?
Hormones regulate body composition, energy use, and glucose handling: estrogen, testosterone, thyroid hormones, and cortisol all affect insulin sensitivity and fat distribution. Imbalances in these hormones can perpetuate metabolic dysfunction. Oxidative stress damages mitochondria and disrupts insulin signaling, which is why antioxidant and mitochondrial-supportive strategies are clinically valuable. Targeted hormone panels—ordered based on symptoms and context—help determine when bioidentical hormone optimization or other interventions are appropriate as adjuncts to core lifestyle therapy. Reducing oxidative stress through antioxidant-rich foods, supplements when indicated, and lifestyle changes supports cellular function and often leads to measurable metabolic improvements.
How Can Natural Strategies Support Blood Sugar Management and Diabetes Reversal?

Natural strategies for blood sugar control combine dietary changes, structured exercise, sleep and circadian alignment, stress reduction, and behavior-change methods to produce lasting improvements in insulin sensitivity and glycemia. Each element contributes differently: diet lowers glycemic load, exercise increases glucose uptake and mitochondrial health, sleep restores hormonal balance, and stress reduction dampens HPA-axis–driven spikes. Putting these elements together with measurable goals—weight milestones, HbA1c targets, fasting glucose improvements—creates predictable timelines for progress and helps both clinicians and patients track objective change. The most effective plans are individualized to a person’s preferences, medical context, and readiness to change.
High-impact lifestyle interventions that consistently support glycemic improvement include these core areas:
- Dietary change: Lower glycemic load and prioritize whole-food patterns to blunt post-meal glucose rises.
- Exercise prescription: Combine resistance and aerobic training to boost insulin-mediated glucose uptake.
- Sleep and circadian hygiene: Maintain regular sleep timing to support metabolic hormones.
- Stress reduction: Practice mindfulness, breathwork, or HRV-guided techniques to reduce cortisol-driven glucose dysregulation.
These approaches work synergistically: diet reduces the glucose burden, exercise increases clearance, and solid sleep and stress habits stabilize the hormonal environment that otherwise resists change.
| Dietary/Lifestyle Protocol | Primary Mechanism | Expected Outcome / Suitability |
|---|---|---|
| Low-carbohydrate diet | Reduces postprandial glycemic load | Fast reductions in fasting and post-meal glucose; best for motivated patients |
| Ketogenic protocol for T2D remission | Sustained carbohydrate restriction, increased ketones | Marked short-term A1c and weight drops; requires clinical supervision |
| Mediterranean diet adapted for diabetes | Anti-inflammatory, high MUFAs and fiber | Improves cardiometabolic risk and glycemic control; broadly sustainable |
| Calorie-restricted/weight-loss programs | Induces negative energy balance and hepatic fat loss | High remission likelihood with sufficient weight loss; needs adherence |
These comparisons help match a patient’s preferences and clinical goals to an appropriate dietary and lifestyle pathway.
If you want clinical support, practitioners using a functional medicine framework translate these evidence-based strategies into individualized plans that combine nutrition coaching, supervised exercise protocols, targeted nutrient repletion, and scheduled monitoring to track remission progress and guide medication changes.
Which Dietary Protocols Are Effective for Type 2 Diabetes Remission?
Several dietary approaches can lower blood sugar and, when paired with weight loss and sustained behavior change, support remission. Low-carbohydrate and ketogenic plans tend to reduce post-meal glucose and drive rapid weight loss with A1c improvement. Mediterranean-style diets emphasize anti-inflammatory foods and are generally easier to maintain long-term while protecting cardiovascular health. Intensive calorie-restricted programs can produce remission by reducing liver and pancreatic fat, especially when started early. Across all approaches, monitor nutrient adequacy, adjust medications safely, and pair food plans with behavioral support for the best long-term outcomes.
What Lifestyle Changes Enhance Insulin Sensitivity and Promote Remission?
Exercise that blends resistance training (2–3 sessions per week) with moderate aerobic activity (about 150 minutes per week) consistently improves insulin sensitivity and body composition. For people short on time, high-intensity interval training is an effective alternative. Sleep—aiming for 7–9 hours nightly with consistent timing—helps normalize leptin, ghrelin, and cortisol. Stress-reduction tools like paced breathing, mindfulness, and brief HRV sessions lower sympathetic drive and cortisol spikes that worsen glycemia. Practical behavioral strategies—breaking changes into small steps, tracking progress, and using regular feedback—increase adherence and support lasting remission.
What Supplements and Herbal Remedies Aid in Managing Type 2 Diabetes?
Targeted supplements can complement core lifestyle changes by supporting insulin signaling, reducing oxidative stress, and improving peripheral glucose uptake. They are adjuncts—not replacements—for diet, exercise, or prescribed medications. Options with clinical support include chromium, magnesium, alpha-lipoic acid (ALA), berberine, and cinnamon; each works through different pathways, such as improving insulin receptor function, supporting cellular energy, or modulating gut-related glucose handling. Safety is essential: supplements may interact with glucose-lowering drugs or affect blood pressure and liver tests, so coordinated monitoring is required. The table below summarizes common choices, mechanisms, dosing ranges, and evidence notes to help clinicians and patients evaluate options.
Below are supplements that support glucose metabolism through specific biochemical actions and evidence levels.
| Supplement | Mechanism of Action | Clinical Effect / Typical Dose / Evidence |
|---|---|---|
| Chromium (picolinate) | Enhances insulin receptor signaling | May modestly improve fasting glucose; common doses 200–1,000 mcg/day; evidence mixed |
| Magnesium (glycinate/oxide) | Cofactor for insulin signaling enzymes | Corrects deficiency and improves insulin sensitivity; typical 200–400 mg elemental/day |
| Alpha-lipoic acid (ALA) | Antioxidant, improves mitochondrial function | Supports neuropathy symptom relief and glycemic markers; usual 300–600 mg/day |
| Berberine | Activates AMPK, reduces hepatic glucose production | Lowers A1c and fasting glucose; typical 500 mg two to three times daily |
| Cinnamon (cassia or Ceylon) | Improves insulin receptor function and glycemic response | Small reductions in fasting glucose; doses 1–3 g/day in studies |
How Do Chromium, Magnesium, and Alpha-Lipoic Acid Support Glucose Metabolism?
Chromium may enhance insulin signaling and improve glucose uptake in some people, especially those with low dietary chromium. Magnesium is an essential cofactor for many enzymes, including those involved in insulin receptor function, and correcting deficiency often improves insulin sensitivity. Alpha-lipoic acid offers antioxidant and mitochondrial support, lowering oxidative stress that impairs insulin signaling and helping with neuropathy symptoms in trials. Typical therapeutic ranges include chromium picolinate (often 200–500 mcg daily), magnesium (200–400 mg elemental daily), and ALA (300–600 mg daily); dosing should account for kidney function, other medications, and baseline nutrient status. Track symptoms and glycemic markers to determine clinical benefit.
What Are the Benefits and Usage of Berberine, Cinnamon, and Other Herbal Supplements?
Berberine lowers glucose largely via AMPK activation, cutting hepatic glucose output and improving peripheral sensitivity; many studies use 500 mg two or three times daily and show short-term A1c benefits comparable to some oral drugs. Cinnamon has modest evidence for lowering fasting glucose and improving insulin sensitivity in certain groups when used at gram-level doses; product quality and species (cassia vs. Ceylon) matter. Other botanicals—bitter melon, fenugreek, gymnema—have emerging data but need more standardized trials. Because herbs can increase hypoglycemia risk when combined with medications, careful dosing and clinician oversight are required.
How Does Dr. Fred Bloem Personalize Functional Medicine Treatment Plans for Diabetes?
Personalized functional medicine care starts with a thorough intake that combines symptom history, lifestyle assessment, and prioritized lab testing to uncover root causes. That diagnostic picture guides sequential, measurable interventions tailored to each patient’s physiology. Testing may include standard glycemic panels plus targeted hormone testing, nutrient assessments, inflammatory markers, and gut profiling when indicated — findings that often reveal treatable contributors such as thyroid problems, sex-hormone imbalances, micronutrient deficits, or dysbiosis. Treatment sequencing commonly begins with nutrition and activity prescriptions, adds targeted supplements and micronutrient repletion, considers bioidentical hormone optimization when appropriate, and uses IV or regenerative therapies selectively. Frequent monitoring and stepwise adjustments keep care focused on restoring function rather than only managing symptoms. The practice emphasizes measurable goals (HbA1c targets, weight milestones) and patient education to support sustainable change.
| Diagnostic / Therapy | Use / Rationale | Timing / Monitoring Metrics |
|---|---|---|
| Advanced metabolic panel (A1c, fasting, OGTT) | Baseline glycemic staging | Repeat A1c every 3 months until stable |
| Hormone evaluation (thyroid, sex hormones) | Identify hormonal drivers of insulin resistance | Monitor symptom response and hormone levels every 3–6 months |
| Nutrient testing (magnesium, vitamin D, B12) | Detect deficiencies that impair metabolism | Recheck after supplementation at 8–12 weeks |
| Gut/stool analysis | Detect dysbiosis and inflammation | Reassess symptoms and targeted markers after interventions |
| Nutrition + exercise plan | Core intervention to reduce hepatic fat and improve insulin sensitivity | Track weight, fasting glucose, and A1c on defined schedule |
| Targeted supplements and IV nutrient support | Address biochemical deficits and accelerate recovery | Monitor for interactions and clinical response regularly |
This structured workflow helps patients know when to expect objective changes and how clinical decisions will be made.
If you’re interested in personalized, root-cause care, Dr. Fred Bloem / Internal Healing and Wellness MD in Kensington, MD offers a functional medicine approach that blends advanced diagnostics, individualized nutrition planning, bioidentical hormone evaluation, regenerative options, and IV therapies when appropriate. The practice focuses on individualized sequencing, outcome tracking, and patient education to improve metabolic health and safely reduce medication burden when possible.
What Diagnostic and Therapeutic Approaches Are Used in Personalized Care?
Personalized care follows a stepwise algorithm: confirm glycemic status, screen for common contributors (thyroid, sex hormones, micronutrients), evaluate gut and inflammatory markers as indicated, and prioritize non-pharmacologic interventions before adjunctive therapies. Treatment choices target the dominant drivers — for example, weight-focused nutrition and exercise for obesity-driven insulin resistance, hormone optimization for hypogonadism or estrogen deficiency, or gut-directed therapies when dysbiosis is identified. Monitoring uses objective metrics (A1c, fasting glucose, weight, symptom scores) at predefined intervals to guide escalation or tapering, ensuring care is evidence-informed and customized.
How Are Bioidentical Hormone Replacement and Regenerative Therapies Integrated?
Bioidentical hormone replacement is considered when clinical symptoms and labs indicate hormone insufficiency that contributes to metabolic dysfunction. The goal is to restore physiologic hormone levels to improve body composition, energy, and insulin sensitivity while monitoring for safety. Regenerative therapies (for example, joint-supportive options) are used as adjuncts to improve function and increase capacity for movement, which indirectly supports metabolic health. Integration is conservative and evidence-informed: hormones and regenerative options are added only when appropriate, with contraindication screening and ongoing monitoring to align treatments with metabolic goals and minimize risk.
How Can Holistic Prevention Strategies Reduce the Risk of Developing Type 2 Diabetes?
Primary prevention centers on improving diet, increasing regular physical activity, maintaining a healthy weight, optimizing sleep, and lowering chronic stress and toxin exposures to reduce lifetime risk of type 2 diabetes. Early screening for prediabetes and timely lifestyle interventions often halt progression; small, consistent changes—better food choices, daily movement, and improved sleep—produce measurable metabolic gains within months. Prevention is most effective when matched to risk level: routine counseling for average-risk adults, structured programs for people with prediabetes, and intensive multidisciplinary support for high-risk individuals. Addressing environmental toxin exposures as part of a comprehensive plan can reduce mitochondrial stress and support long-term resilience.
Specific lifestyle and nutrition actions that support prevention include the following evidence-based practices.
- Key preventive practices include:
Adopt a whole-food, moderate-carbohydrate or Mediterranean-style eating pattern: Emphasize fiber, lean protein, and healthy fats.
Achieve and maintain regular physical activity: Include both aerobic and resistance training throughout the week.
Prioritize sleep and circadian alignment: Keep consistent sleep timing to support metabolic hormones.
Implement stress-management routines: Daily brief mindfulness or breathing exercises reduce HPA-axis activation.
What Lifestyle and Nutritional Practices Support Diabetes Prevention?
Sustainable dietary patterns—like a Mediterranean-style plan adapted to lower glycemic load—combined with regular physical activity reduce the likelihood of developing diabetes across populations. Weight-management strategies that pair modest calorie reduction with nutrient-dense foods and resistance training preserve lean mass and improve metabolic rate, enhancing preventive effects. Monitoring weight trends, fasting glucose, and A1c in people with risk factors enables earlier intervention when trends worsen. Move to structured programs or clinical support when simple measures don’t reverse prediabetes or when multiple high-risk features are present.
How Does Stress Management and Detoxification Contribute to Metabolic Health?
Chronic stress activates the HPA axis and raises cortisol, which increases hepatic glucose production and visceral fat, worsening insulin resistance. Short, daily stress-management practices blunt this pathway and improve metabolic outcomes. Detoxification efforts focused on lowering exposure to metabolic disruptors—persistent organic pollutants and endocrine-disrupting chemicals—help preserve mitochondrial function and hormone balance, though clinical detox should be individualized and evidence-based. Safe, practical steps include improving indoor air and water quality, choosing low-toxin food preparation, and supporting natural detox pathways with nutrition and sleep. If toxin burden is suspected, targeted testing and supervised interventions may be appropriate.
For people who want a coordinated prevention plan that addresses root causes and builds measurable metabolic resilience, Dr. Fred Bloem / Internal Healing and Wellness MD in Kensington, MD offers integrative prevention-focused care combining nutrition coaching, lifestyle support, and targeted diagnostics to lower diabetes risk through personalized strategies.
Frequently Asked Questions
What lifestyle changes can significantly reduce the risk of developing Type 2 diabetes?
The most impactful changes are a whole-food diet (plenty of vegetables, lean protein, healthy fats), regular physical activity that includes both aerobic and resistance work, weight management, consistent sleep, and daily stress-reduction practices. Early screening for prediabetes and timely lifestyle programs also prevent progression. Small, sustained changes add up — they’re often more effective than dramatic short-term fixes.
How can stress management techniques improve insulin sensitivity?
Stress practices like mindfulness, meditation, and deep breathing lower chronic cortisol exposure. High cortisol promotes insulin resistance and raises blood sugar; reducing it helps stabilize hormonal balance and improves glucose metabolism. Regular, brief practice (even 5–10 minutes daily) produces measurable benefits for both mental well-being and metabolic health.
What role does sleep play in managing Type 2 diabetes?
Sleep is fundamental. Poor or short sleep disrupts hormones such as leptin, ghrelin, and cortisol, increasing appetite, worsening insulin resistance, and raising blood sugar. Aiming for 7–9 hours per night with consistent timing supports metabolic recovery, better glucose control, and weight management. Simple steps — a regular bedtime, reducing evening screens, and a cool, dark bedroom — improve sleep quality.
Are there specific dietary patterns that are particularly effective for diabetes prevention?
Yes. The Mediterranean-style diet, rich in fiber, healthy fats, and lean protein, is strongly supported for diabetes prevention. Low- or moderate-carbohydrate patterns can also be effective for blood sugar control. Choose the approach that fits your health needs and that you can sustain long term; sustainability is a key predictor of success.
How can supplements aid in the management of Type 2 diabetes?
Supplements may support insulin signaling, reduce oxidative stress, and improve glucose handling — examples include chromium, magnesium, and alpha-lipoic acid. They’re adjuncts rather than primary treatments and should be used under clinical supervision because of potential interactions with medications and individual safety considerations. Monitoring is important to evaluate benefit and avoid harm.
What is the importance of personalized care in managing Type 2 diabetes?
Personalized care tailors treatments to a person’s unique biology, lifestyle, and goals. By combining detailed history, targeted labs, and prioritized interventions that address root causes, personalized plans improve adherence and outcomes and increase the chance of meaningful metabolic improvement — including possible remission when appropriate.
Conclusion
Managing Type 2 diabetes through a holistic, functional medicine lens can produce meaningful improvements in metabolic health and quality of life. By addressing root causes — inflammation, gut health, hormones, and lifestyle — people can gain better blood sugar control and, in some cases, achieve remission. Practical, personalized strategies (diet, movement, sleep, stress management, and targeted supplements) empower patients to reclaim their health. If you’d like individualized guidance, consider exploring our functional medicine services to build a tailored plan that fits your goals.



