Integrative Care for Chronic Fatigue Syndrome (ME/CFS): Functional, Holistic Approaches to Restore Lasting Energy
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex, multisystem condition marked by profound, persistent exhaustion, post-exertional malaise, cognitive slowing, and unrefreshing sleep that do not resolve with rest. Integrative care aims to identify and treat the biological drivers beneath these symptoms so patients can rebuild sustained energy and daily function.
This article describes how functional and holistic medicine address root causes — including mitochondrial dysfunction, immune imbalance, gut microbiome disruption, hormonal issues, and toxicant exposure — and how targeted therapies such as IV nutrient support, detox protocols, ozone therapy, bioidentical hormone optimization, and lifestyle changes work together to improve cellular resilience and immune balance.
We outline practical diagnostic steps, evidence-informed treatment options, nutrition and behavioral strategies, and emerging approaches like Low Dose Naltrexone and photobiomodulation that current research suggests may lessen symptom burden for some patients.
The sections that follow define clinical criteria and diagnostic pathways, summarize common root causes with testing guidance, review integrative therapies and their mechanisms, offer lifestyle and supplement recommendations, highlight promising new treatments, and explain why a patient-centered, root-cause approach often yields better long-term outcomes.
Throughout, we include searchable phrases such as integrative fatigue solutions, mitochondrial support for chronic fatigue, IV vitamin therapy chronic fatigue, and ME/CFS integrative care to help readers find practical, functional pathways to recovery.
What Is Chronic Fatigue Syndrome and How Is It Diagnosed?
ME/CFS is diagnosed when disabling fatigue lasts six months or longer, significantly reduces activity, cannot be explained by another condition, and is accompanied by hallmark features like post-exertional malaise and unrefreshing sleep. There is no single laboratory test; diagnosis depends on careful clinical criteria and targeted testing.
Clinicians assess symptom clusters, onset patterns, and objective findings while using routine labs and, when indicated, specialized testing to evaluate inflammation, thyroid and adrenal function, and metabolic or mitochondrial health.
Recognizing ME/CFS early reduces delays in care and allows earlier introduction of mitochondrial support, immune modulation, and activity pacing to limit decline.
A thoughtful diagnostic strategy balances ruling out reversible causes with identifying treatable contributors; patients benefit from clinicians experienced in integrative, functional evaluations for complex fatigue.
Below is a concise diagnostic checklist to clarify next steps for patients and clinicians.
What Are the Key Symptoms and Impact of ME/CFS on Daily Life?
ME/CFS produces a predictable cluster of symptoms that limit work, relationships, and everyday activities by lowering available energy and recovery capacity. Typical features include profound fatigue that doesn’t improve with rest, post-exertional malaise (PEM) where physical or cognitive effort worsens symptoms, unrefreshing sleep, cognitive dysfunction often called “brain fog,” and autonomic problems such as orthostatic intolerance. Symptoms can fluctuate and are commonly triggered or worsened by infections or environmental stressors.
Functional impact ranges from reduced work hours to full-time disability for many people. Quality-of-life studies show declines comparable to other serious chronic illnesses. Appreciating the full scope of symptoms helps prioritize testing and tailor treatment plans that focus on energy restoration and careful pacing.
The next subsection explains why diagnosing ME/CFS remains difficult despite clear clinical patterns.
- Key symptom clusters include:
Post-exertional malaise: worsening of symptoms after exertion.
Unrefreshing sleep: sleep that does not restore function.
Cognitive dysfunction: slowed thinking, memory lapses, trouble concentrating.
Autonomic symptoms: lightheadedness, orthostatic intolerance, palpitations.
These core symptoms guide focused assessment and a treatment sequence that prioritizes stabilization before increasing activity tolerance.
Why Is Diagnosing Chronic Fatigue Syndrome Challenging?
ME/CFS is challenging to diagnose because no single lab or imaging study confirms it. Clinicians rely on a detailed history, recognition of symptom patterns—particularly PEM—and selective testing to exclude mimics such as untreated hypothyroidism, sleep apnea, major depressive disorder, and cardiopulmonary disease.
Overlap with conditions like fibromyalgia, POTS, and post-viral syndromes contributes to underdiagnosis and misdiagnosis. Epidemiologic data through 2023 show many patients face diagnostic delays measured in years.
Spotting red flags and using targeted labs—CBC, metabolic panel, thyroid tests, inflammatory markers, and tailored immune or mitochondrial assays when indicated—helps distinguish primary ME/CFS from other treatable conditions.
Early referral to practitioners skilled in integrative and functional evaluation increases the chance of finding reversible contributors and starting individualized treatment. This diagnostic complexity leads into a review of common root causes clinicians address.
What Are the Root Causes of Chronic Fatigue Syndrome?
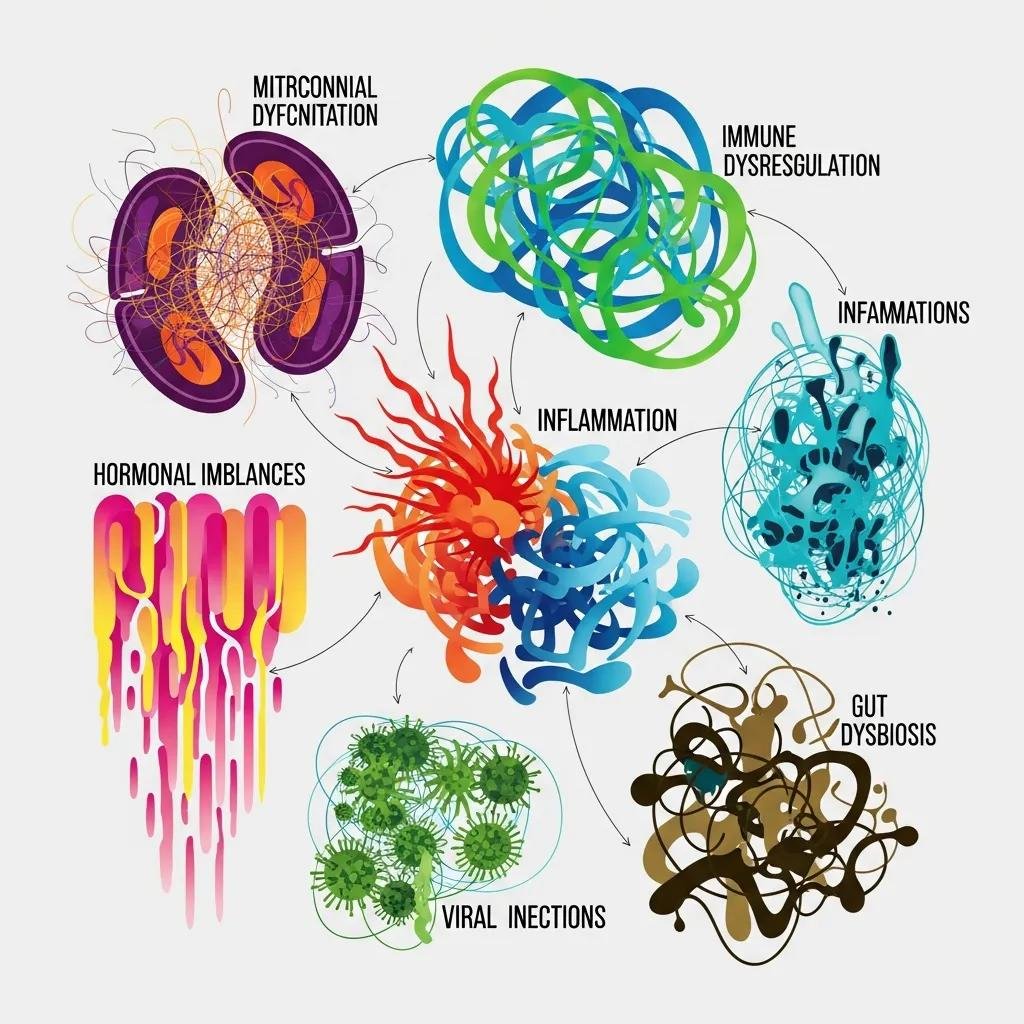
ME/CFS most commonly reflects interacting biological processes — mitochondrial dysfunction, immune dysregulation with chronic inflammation, gut microbiome imbalance, persistent infectious triggers, endocrine abnormalities, and environmental toxicant exposure — that together impair cellular energy production and reduce resilience to stress.
Knowing these root causes helps determine which targeted tests (for example, lactate, CoQ10, specialized immune panels, stool microbiome analysis, viral serologies) and which interventions (mitochondrial cofactors, antimicrobials when indicated, detoxification, hormone optimization) are appropriate for each patient.
Recent work supports addressing multiple interacting drivers rather than treating single symptoms in isolation to achieve better functional gains.
The table below links common root causes to underlying pathophysiology and the clinical signs or tests clinicians use to prioritize assessments.
Different root causes map to specific mechanisms and point toward targeted testing that guides therapy selection.
| Root cause | Pathophysiology | Clinical signs / diagnostic tests |
|---|---|---|
| Mitochondrial dysfunction | Reduced ATP production; increased oxidative stress | Severe fatigue, post-exertional lactate rise, low CoQ10 or carnitine, lactate/pyruvate assays |
| Immune dysregulation | Persistent cytokine activation; possible autoimmunity | Cytokine panels, CRP/ESR, autoantibody testing, infection serologies |
| Gut dysbiosis | Altered microbiome driving systemic inflammation | Stool microbiome analysis, GI symptoms, markers of gut permeability |
| Viral / infectious triggers | Ongoing viral reservoirs or immune activation | Selective viral PCR/serology, history of infection-triggered onset |
| Hormonal imbalance | Thyroid, sex hormone, or HPA-axis dysfunction | Thyroid panel, sex hormone testing, cortisol rhythm or adrenal assessments |
| Toxicant burden | Environmental toxins that impair mitochondria and immunity | Exposure history, targeted heavy metal or organic toxin testing |
This framework shows why an individualized assessment is central to effective integrative care and how therapies are matched to specific mechanisms.
The next subsection looks more closely at mitochondrial dysfunction because it is a frequent contributor to fatigue.
How Does Mitochondrial Dysfunction Contribute to Fatigue?
When mitochondria are impaired, ATP production falls and reactive oxygen species rise. The result is less cellular energy, reduced muscle endurance, and the profound fatigue that characterizes ME/CFS.
Clinically, patients may demonstrate exercise intolerance with abnormal post-exertional lactate increases, low CoQ10 or carnitine levels, and symptoms that align with reduced oxidative phosphorylation efficiency. Targeted testing can reveal these patterns.
Treatments that supply mitochondrial cofactors — CoQ10, NAD precursors, L‑carnitine — and interventions that lower oxidative stress can improve cellular energy metabolism when they are matched to identified deficits.
For some patients, IV nutrient therapies that deliver mitochondrial substrates and antioxidants directly into the circulation can bypass absorption limits and produce measurable gains in energy and mental clarity.
Next we explore how gut and immune drivers often perpetuate mitochondrial stress.
What Role Do Gut Microbiome Imbalances and Immune Dysregulation Play?
Imbalances in the gut microbiome can drive systemic inflammation through increased intestinal permeability, altered metabolite production, and immune activation. This gut–immune–brain axis is implicated in persistent symptoms and cognitive dysfunction in ME/CFS.
Stool testing may show dysbiosis, loss of beneficial species, or pathogen overgrowth that correlate with systemic inflammatory findings. Interventions such as dietary changes, targeted probiotics, and selective antimicrobials or immunomodulatory treatments aim to rebalance the microbiome.
Immune dysregulation — low-level chronic cytokine elevation, altered T-cell responses, or autoantibodies — can sustain fatigue and block recovery. Immunologic panels help identify treatable patterns.
Addressing gut and immune contributors often lowers systemic inflammation and secondarily improves mitochondrial function, supporting a combined, sequential treatment strategy that targets multiple systems.
With root causes mapped, the next section describes the integrative therapies commonly used in clinical care plans.
Which Integrative Therapies Does Dr. Fred Bloem Offer for Chronic Fatigue?

We use diagnostic-guided, mechanism-focused therapies to target the drivers outlined above. Typical options include autonomic response testing, IV nutrient therapy, individualized detox protocols, ozone/biooxidative therapy, bioidentical hormone optimization, specialized lab testing, and a structured Root Cause Protocol that sequences care safely.
Each intervention is selected to address a specific mechanism — IV nutrients deliver mitochondrial cofactors and antioxidants, ozone therapy may modulate oxidative stress and immune signaling, BHRT treats endocrine contributors, and detoxification reduces toxin-related mitochondrial and immune disruption — and protocols are individualized based on testing and tolerance.
The table below compares common therapies by mechanism and typical clinical indication to help patients and clinicians understand how these options fit into a personalized plan.
| Therapy | Mechanism (attribute) | Clinical benefit / typical indication |
|---|---|---|
| IV nutrient therapy (e.g., NAD+, vitamin C, B-complex) | Delivers cofactors and antioxidants directly into circulation | Rapid mitochondrial support; improved energy and cognitive clarity for selected patients |
| Ozone / biooxidative therapy | Modulates oxidative stress and immune signaling | May reduce chronic inflammation and support immune regulation in appropriate candidates |
| Bioidentical hormone replacement (BHRT) | Restores physiologic hormone balance | Improves energy, sleep, mood, and metabolic resilience when hormone deficits are present |
| Detoxification protocols | Enhances elimination of environmental toxicants | Lowers toxicant burden that can impair mitochondrial and immune function |
| Autonomic response testing | Clinical assessment of autonomic and functional imbalances | Helps guide individualized sequencing of therapies and ongoing monitoring |
How Do IV Nutrient Therapies and Detoxification Support Recovery?
IV nutrient therapies supply mitochondrial cofactors, antioxidants, and electrolytes directly into the bloodstream, bypassing intestinal absorption limits to more rapidly support cellular metabolism and immune resilience. Common formulations include NAD precursors, vitamin C, magnesium, and B-complex nutrients chosen to address laboratory-identified deficits.
Many patients experience quicker relief of cognitive fog and energy with IV therapy compared with oral supplements alone, particularly when malabsorption or severe fatigue limit oral intake. Safety screening and baseline labs help minimize risks.
Detoxification plans are tailored and may use binders, targeted nutrients, and strategies to support hepatic phase I/II pathways, aiming to reduce toxin-driven mitochondrial and immune impairment.
Safety and personalization are essential: sequencing, monitoring labs, and paced interventions prevent setbacks while maximizing therapeutic benefit.
Because these services require clinical expertise, patients often seek practices that combine in-depth diagnostics with a broad therapeutic toolkit.
Internal Healing and Wellness MD in Kensington, MD — led by Dr. Fred Bloem — offers the integrative services described here, emphasizing individualized protocols and comprehensive testing to match therapies to root causes. The practice focuses on longer, patient-centered visits, root-cause resolution, and coordinated care to support recovery.
What Benefits Do Bioidentical Hormone Replacement and Ozone Therapy Provide?
Bioidentical hormone replacement (BHRT) restores physiologic hormone levels and supports thyroid and adrenal balance. When hormone deficits contribute, BHRT can reduce fatigue, improve sleep, stabilize mood, and boost metabolic efficiency.
BHRT works best when guided by objective testing and careful dose titration, with ongoing monitoring to assess response and safety. Correcting endocrine contributors often enhances benefits from mitochondrial and immune-focused therapies.
Ozone therapy, administered in clinical settings by trained practitioners, is proposed to modulate oxidative stress, improve tissue oxygenation, and influence immune signaling — mechanisms that may lower chronic inflammation contributing to fatigue.
Evidence is evolving; careful patient selection and safety assessment are critical. When integrated into a coordinated root-cause plan, these modalities can complement nutritional, detox, and lifestyle interventions.
The next section covers lifestyle and nutritional strategies patients can use alongside clinical therapies.
How Can Lifestyle and Nutritional Strategies Enhance Chronic Fatigue Treatment?
Lifestyle and nutrition are foundational adjuncts that support medical therapies by lowering systemic inflammation, improving mitochondrial function, optimizing sleep and stress response, and fostering microbiome health. Combined with targeted clinical interventions, these steps increase the chance of sustained improvement.
Core elements include anti-inflammatory eating patterns, evidence-supported supplements for mitochondrial and immune support, structured sleep hygiene and stress-reduction practices, and pacing/activity management to avoid PEM while gradually rebuilding capacity.
Practical measures are chosen based on individual testing and tolerance; careful sequencing prevents overexertion during early recovery.
The table below summarizes key interventions, their biological targets, and practical recommendations so patients and clinicians can incorporate them into personalized care.
| Intervention | Biological target | Practical recommendation / dosage |
|---|---|---|
| Anti-inflammatory diet | Reduces systemic inflammation | Prioritize whole foods, omega‑3s, and fiber; limit ultra-processed foods and added sugars |
| CoQ10 supplementation | Supports mitochondrial electron transport | Typical range 100–300 mg/day, adjusted to labs and tolerance |
| NAD/NADH precursors | Supports cellular energy pathways | Use under clinical supervision; oral or IV routes as appropriate |
| Magnesium | Supports cellular energy and neuromuscular function | 200–400 mg/day of glycinate as tolerated, individualized |
| Vitamin D | Immune modulation | Supplement to maintain serum 25(OH)D in target range per clinician guidance |
Combining these interventions with medical treatments creates the biochemical environment needed for cellular recovery and improved resilience.
The next subsections go into supplements and behavioral strategies in more detail.
Which Supplements and Anti-Inflammatory Diets Support Energy and Wellness?
A focused supplement plan can support mitochondrial function, reduce oxidative stress, and correct common micronutrient deficits. Evidence-backed options include CoQ10, NADH or NAD precursors when appropriate, magnesium glycinate for neuromuscular and mitochondrial support, B‑complex vitamins for energy metabolism, and vitamin D for immune balance.
Dosing should be individualized using laboratory data and clinical response. Clinicians tailor regimens to avoid unnecessary polypharmacy while addressing documented deficiencies and functional needs.
An anti-inflammatory eating pattern emphasizes whole foods, lean protein, healthy fats (including omega‑3s), abundant vegetables and fiber to support the microbiome, and minimization of ultra‑processed foods and added sugars. Personalization is important: food sensitivities, GI symptoms, and metabolic factors guide specific choices and timing.
With nutrition in place, behavioral strategies further support recovery.
- Supplements commonly used in ME/CFS care:
CoQ10: supports mitochondrial electron transport.
NAD/NADH precursors: support cellular energy pathways under clinical supervision.
Magnesium (glycinate): supports neuromuscular function and sleep quality.
B-complex: supports energy metabolism and nervous system health.
Vitamin D: supports immune regulation and overall wellness.
These approaches are paired with testing and monitoring to optimize safety and effectiveness.
What Are Effective Sleep Hygiene, Stress Management, and Pacing Techniques?
Good sleep habits and stress management restore restorative sleep architecture and improve autonomic balance, which supports energy metabolism and daytime function. Practical steps include consistent sleep and wake times, reducing evening stimulants and blue light, creating a calming pre-sleep routine, and evaluating and treating comorbid sleep disorders when present.
Stress-reduction practices that rebalance the autonomic nervous system — guided breathing, gentle mindfulness, and graded relaxation — lower sympathetic overactivation and may reduce inflammatory signals linked to fatigue.
Pacing is essential: stay within your personal energy envelope to avoid PEM. Use activity logs, break tasks into short segments with scheduled rest, and only increase activity slowly when stability is maintained.
Clinicians combine these behavioral strategies with medical therapies to maximize gains and minimize setbacks. The next section reviews emerging therapies and innovations in ME/CFS care.
- Pacing and behavioral tips:
Track activity: keep simple logs to identify PEM triggers.Chunk tasks: break activities into short segments with planned rest.Gentle movement: prioritize low-intensity mobility and flexibility as tolerated.
What Emerging Treatments and Innovations Are Available for ME/CFS?
New and evolving treatments include immunomodulatory therapies, Low Dose Naltrexone (LDN), photobiomodulation (PBM), and advances in diagnostics such as AI-assisted pattern recognition and specialized biomarker panels designed to better phenotype patients and match therapies to mechanisms.
Research through 2023 highlights LDN for immune modulation and PBM for mitochondrial stimulation as promising approaches with growing clinical interest. Translational work aims to identify reproducible biomarkers to reduce diagnostic delays and enable more precise therapies.
Availability, cost, and regulatory issues vary across treatments, and careful patient selection and monitoring are essential when applying these innovations.
The next two subsections summarize the rationale and current clinical context for LDN and PBM so patients and clinicians can weigh potential benefits against existing evidence.
How Does Low Dose Naltrexone Help Reduce Inflammation?
Low Dose Naltrexone (LDN) is thought to produce immunomodulatory effects by briefly blocking opioid receptors at low doses, which may trigger a compensatory rise in endorphins and downregulate pro-inflammatory cytokines. This mechanism could help modulate immune activation seen in ME/CFS.
Smaller clinical reports and pilot trials through 2023 show symptom improvement for some patients with chronic inflammatory and pain conditions, but larger randomized trials in ME/CFS are still needed to define who benefits most and to refine dosing protocols. LDN is typically used under clinical supervision.
LDN is generally well tolerated but requires review of contraindications and potential drug interactions. Clinicians monitor symptoms and adjust treatment as part of a broader care plan.
When used, LDN is one component among multiple strategies aimed at reducing immune-driven fatigue.
What Is Photobiomodulation Therapy and Its Role in Fatigue Management?
Photobiomodulation (PBM) uses red and near‑infrared light to stimulate mitochondrial chromophores, increase ATP production, and reduce oxidative stress — cellular effects that provide a rationale for PBM in fatigue and pain syndromes.
Clinical studies in related conditions report improvements in pain, function, and fatigue. Treatment parameters — wavelength, energy density, and session frequency — are tailored to the target tissue and patient tolerance. When devices are used correctly, safety profiles are favorable.
PBM is usually part of a broader rehabilitation plan; patients may notice incremental gains across multiple sessions as mitochondrial function and tissue repair respond.
Practical considerations include device access, cost, and coordination with other treatments. Next we explain why choosing an experienced, patient-centered clinician matters when navigating these options.
Why Choose Dr. Fred Bloem for Integrative Chronic Fatigue Syndrome Care?
Successful ME/CFS care depends on thorough diagnostic evaluation, individualized sequencing of therapies, and ongoing monitoring as physiology changes. Dr. Fred Bloem’s practice emphasizes these elements through patient-centered, root-cause-focused care designed to address the full complexity of chronic fatigue.
Internal Healing and Wellness MD offers a comprehensive suite of integrative services — functional and holistic medicine, autonomic response testing, IV nutrient therapy, detox protocols, ozone therapy, bioidentical hormone optimization, regenerative options, supportive oncology care, and a structured Root Cause Protocol — delivered in longer visits that allow detailed intake, education, and collaborative planning.
The practice operates outside traditional insurance panels but provides coding information for possible out‑of‑network reimbursement. Patient education and shared decision-making are central to every individualized care plan.
For patients seeking a clinic that combines diagnostic depth with therapeutic breadth, working with experienced practitioners who prioritize root-cause resolution and wellness-focused outcomes can shorten diagnostic delays and improve long-term function.
How Does Patient-Centered Care and Root Cause Resolution Improve Outcomes?
Patient-centered care begins with a thorough intake and timeline reconstruction to identify triggers and prioritize testing. Targeted labs and functional assessments then guide an individualized protocol that tackles the most relevant biological drivers.
Typical workflow includes an extended initial evaluation, ordering of baseline and specialty testing, a coordinated treatment plan that may combine nutritional, detox, hormonal, IV, or biooxidative therapies as indicated, and scheduled follow-ups for monitoring and dose adjustments. This staged approach reduces the risk of over-treatment and improves tolerability.
Outcomes track symptom reduction, increased activity tolerance, restored sleep quality, and improved quality of life. Clinicians follow objective lab changes along with patient‑reported outcomes to guide ongoing adjustments.
This structured, iterative process contrasts with symptom-only approaches and raises the likelihood of durable improvement when multiple interacting contributors are treated.
What Do Patient Testimonials Reveal About Treatment Success?
Patient stories frequently describe regained energy, clearer thinking, better sleep, and a return to meaningful daily activities after comprehensive, personalized care that addressed biochemical deficits alongside lifestyle factors.
Individual results vary, and objective measures are used for monitoring, but testimonials consistently highlight the value of longer visits, clear education, careful sequencing of therapies, and coordinated multidisciplinary support in producing measurable gains.
Full patient stories and reviews are available through clinic resources for those seeking deeper case-level detail. Aggregated themes suggest that combining mitochondrial support, immune modulation, and lifestyle optimization often provides a synergistic pathway to recovery for many patients.
These real-world outcomes reinforce the benefit of choosing experienced integrative providers to navigate the complexity of chronic fatigue care.
Frequently Asked Questions
What lifestyle changes can help manage Chronic Fatigue Syndrome?
Managing ME/CFS usually requires meaningful lifestyle adjustments. Key steps include adopting an anti‑inflammatory diet focused on whole foods, omega‑3s, and fiber while cutting processed foods and excess sugar. Gentle, regular movement can help but must be balanced with rest to avoid PEM. Consistent sleep routines and stress‑reduction practices such as mindfulness or gentle yoga also support recovery and overall well‑being.
How can I find a qualified practitioner for integrative ME/CFS care?
Look for clinicians who specialize in functional and integrative medicine and have experience diagnosing and treating complex fatigue syndromes. Recommendations from support groups, trusted online directories, or local wellness clinics can help. Ask about their diagnostic approach, testing depth, and how they sequence treatments to ensure their style matches your needs and preferences.
What role does nutrition play in the treatment of Chronic Fatigue Syndrome?
Nutrition is a cornerstone of ME/CFS care: it supplies nutrients that support mitochondrial function and lowers inflammation. A balanced diet rich in antioxidants, vitamins, and minerals can ease symptoms. Targeted supplements such as CoQ10 and magnesium may help when appropriate. Personalizing diet to sensitivities and metabolic needs enhances energy and complements medical treatments.
Are there any risks associated with IV nutrient therapy?
IV nutrient therapy can offer benefits but carries risks, including allergic reactions, infection at the infusion site, or electrolyte imbalances if not carefully monitored. Thorough screening, administration by trained clinicians, and regular lab follow-up reduce risks and help ensure the therapy is safe and appropriate for each person.
What is the importance of patient-centered care in ME/CFS treatment?
Patient-centered care matters because ME/CFS is heterogeneous. A collaborative relationship between patient and clinician enables tailored plans that address root causes rather than just symptoms. Prioritizing patient preferences and clear education improves adherence, outcomes, and overall quality of life.
How do emerging treatments like Low Dose Naltrexone and photobiomodulation work?
LDN may modulate immune activity by transiently blocking opioid receptors and altering cytokine signaling, while PBM uses specific light wavelengths to stimulate mitochondrial function and reduce oxidative stress. Both are promising but still under study; they may help some patients when integrated into a comprehensive, individualized treatment plan.
Conclusion
Integrative care for ME/CFS uses a multifaceted, root‑cause approach to restore energy and improve daily function. By combining targeted therapies — such as IV nutrient support, detoxification, hormone optimization — with personalized lifestyle and nutrition strategies, many patients see meaningful gains in resilience and quality of life. If you’re ready to explore a tailored plan, our team can help you take practical steps toward regaining vitality.



